Optical thermometry and its new, nonlinear variant
Temperature, an essential physical quantity, is commonly and extensively utilized in everyday life, research as well as industrial manufacturing. A wide variety of thermometers of different construction is built on various principles of temperature sensing. A lot of them are in commercial production, and the market of temperature sensing is worth millions of dollars. The most frequently used thermometers are those made of glass that has liquid in them as well as thermocouples, thermistors, as well as resistance thermometers. However, the use of traditional thermometers in various scenarios, including tiny objects, moving objects, biological tissues, severe and hazardous environments, are very limited. Fortunately, the optical thermometry methods, and its new variant, nonlinear optical thermometry effectively solves these issues.
Let us provide some background. In recent times, non-contact optical thermometry has attracted significant attention in the field of inorganic compounds like silicates, oxides and, fluorides, and others. Basically, these compounds withstand really high temperatures and photobleaching processes, have so-called low phonon energy, and could show high light emission in comparison to organic compounds. Actually, optical thermometry can be successfully realized by employing temperature-dependent optical parameters, including the relative ratio of fluorescence integral intensities, lifetime, bandwidth, to name the most important ones, although there are other options as well. Self-calibrated optical thermometers that utilize the fluorescence intensity ratio method are the most reliable method due to their independence of loss of energy as well as external perturbations and instabilities in the concentration of excitation sources. Thus, the technique of fluorescence intensity ratio helps to achieve high reliability in measurement and their precision. It must be stressed that fluorescence intensity can be successfully used in both, linear and nonlinear optical thermometry, but excitation pathways are slightly different (see below).
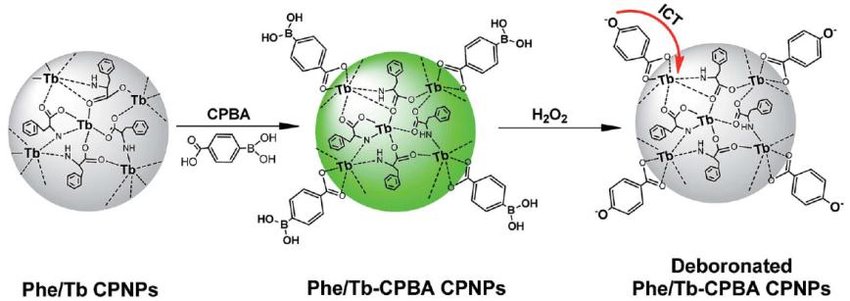
In general, the variation of the intensity ratio of fluorescence is due to the redistribution of electrons that is associated with emission sensitivity of lanthanide ions such as Tb3+ and Eu3+, as well as the transition metal building units, for example, Mn4+. Particularly, doped materials with lanthanide ions, including the famous class of coordination polymers and their subclass of metal-organic frameworks, exhibit multicolor emission, narrow emission bands, longer fluorescence lifetimes, and a significant Stokes shift. The favorable spectroscopic properties of lanthanide centers are due to the non-existent nature of transitions between 4f-4f, as well as the shielding and obscuring of electrons in 4f by 5s orbitals and also the 5p orbitals in a wide palette of available ions.
Indeed, the aforementioned coordination polymers are a not-so-new class of materials (e.g. in the context of classic luminescence properties), but certainly, gain popularity as temperature-sensitive materials. In the past, they have received lots of attention because of their potential to be functional in fields that deal with gas storage luminescence as well as nonlinear optics (second harmonic generation being the most widespread), magnetism, and many more. At present, a range of coordination polymers comprised of transition metals f-block metal ions, and alkaline earth metals have been created. In addition, the controlled synthesizing of the desired lanthanide coordination polymers to detect temperature optically remains a major issue for scientists. This is due to the fact that there are numerous factors that affect the final structure, including the temperature of reaction and solvents, pH values as well as other variables. Furthermore to this, the introduction of second metal building units into the single reaction system could allow the creation of innovative lanthanide coordination polymers with promising properties. At present, numerous luminescent d-d and d-f coordination polymers with intriguing topologies of structure and intriguing properties have been described. But, so far, all the focus has been given to luminescent frameworks that exhibit linear optical thermometry, that is, which employs one-photon excitation paths to achieve thermometric responses.
A completely different approach is the basis for the nonlinear optical thermometry, a new and unique technique to perform spectroscopic temperature readout. In this scenario, the multiphoton absorption process in the near-infrared region is used to excite ultraviolet electronic transitions of linkers building up coordination polymers so that the absorbed energy can be converted into temperature-dependent light emission. That is a new thing to use excitation in the near-infrared range. By contrast, traditional, linear thermometry took advantage of ultraviolet transitions because nearly all coordination polymers contain organic ligand that is a must-have transition in the ultraviolet regions. It is, therefore, no surprise that previously optical thermometry had to make use of excitations in the ultraviolet region because it is a bullet-proof method to obtain ligand-sensitized light emission of a ratiometric mixture of Eu3+ and Tb3+ ions. Sometimes emission of lanthanides can be overlapped with that of ligand fluorescence and theoretically, this can be also used to detect temperature. Without a doubt, this variant could increase even more the attractiveness of new subtypes of nonlinear optical thermometry and can extend to processes such as second-harmonic generation or third-harmonic generation.
You can check the related articles about lanthanide coordination polymers.




